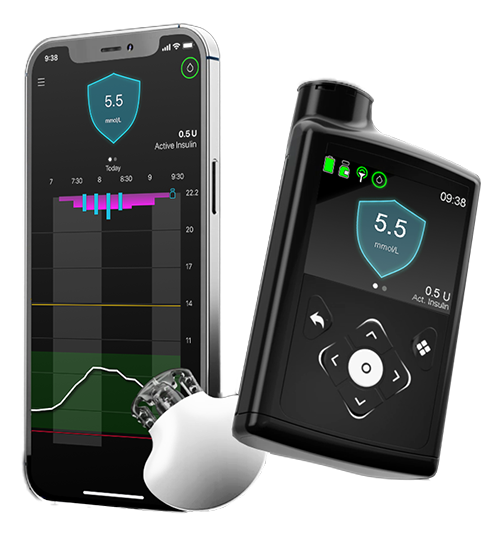
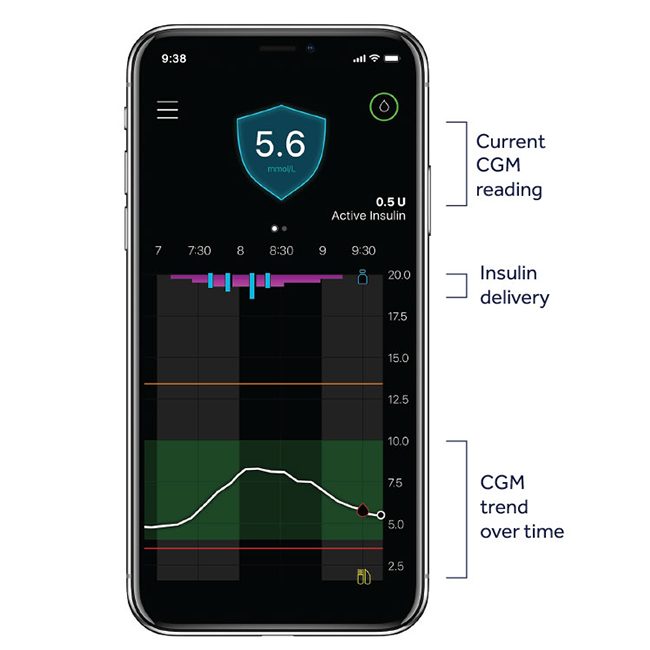
SMARTGUARD™ TECHNOLOGY
The MiniMed™ 780G system^ with advanced SmartGuard™ tech provides you a more effortless way to stabilise your glucose levels. With new auto correction dosing, the system continuously checks your glucose levels and automatically adjusts insulin delivery and corrects highs to help keep you in range*.
On average, users spend
76% of their Time in Range.3
76% of their Time in Range.3

For illustrative purposes only.
1. Carlson, A.L. et al. 97-P- Safety and glycaemic outcomes of the MiniMed™ AHCL System in subjects with T1D. 80th ADA International Conference, June 2020, Chicago+E24
2. Collyns, O. et al. 199-OR- Improved glycaemic Outcomes with MiniMed™ AHCL Delivery. 80th ADA International Conference, June 2020, Chicago
3. Bergenstal, R. M. et al. Safety of a Hybrid-Closed Loop Insulin Delivery System in Patients with Type 1 Diabetes Jama. 2016; 316 (13): 1407 - 1408
Sensor via the NDSS
"Since getting the pump my relationship with diabetes has been a lot easier to manage. What I like most about the pump its there for me, Its working for me… It learns my body and my rhythm, and I can forget about it essentially."
JR, #MedtronicChampion
Individual results may vary. Thoughts are his own.
The system is for type 1 ages 7 and over. Prescription required.
WARNING: Do not use SmartGuard™ feature for people who require less than 8 units or more than 250 units of insulin/day. See bit.ly/780gRisks.
The system is for type 1 ages 7 and over. Prescription required.
WARNING: Do not use SmartGuard™ feature for people who require less than 8 units or more than 250 units of insulin/day. See bit.ly/780gRisks.
"I absolutely love the MiniMed™ 780G! My diabetes is the best it's been in such a long time."
Lily, #MedtronicChampion
Individual results may vary. Thoughts are his own.
The system is for type 1 ages 7 and over. Prescription required.
WARNING: Do not use SmartGuard™ feature for people who require less than 8 units or more than 250 units of insulin/day. See bit.ly/780gRisks.
The system is for type 1 ages 7 and over. Prescription required.
WARNING: Do not use SmartGuard™ feature for people who require less than 8 units or more than 250 units of insulin/day. See bit.ly/780gRisks.
Privacy Statement
By providing your personal information, you agree to allow Medtronic to use this information to communicate with you in the future, including information about products, services, events, and programs. Medtronic will not sell, rent, or otherwise distribute your name and any personally identifiable information outside of Medtronic. Medtronic will use your information in accordance with the Medtronic privacy policy.
ALWAYS FOLLOW THE DIRECTIONS FOR USE.
The MiniMed 780G insulin pump is indicated for use by patients age 7-80 years with Type 1 diabetes, whose total daily dose of insulin is 8 units per day or more. The MiniMed 780G system is intended for the continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts. The system is also intended to continuously monitor glucose values in the fluid under the skin. The MiniMed 780G system includes SmartGuard technology, which can be programmed to provide an automatic adjustment of insulin delivery based on continuous glucose monitoring (CGM) and can suspend the delivery of insulin when the SG value falls below, or is predicted to fall below, predefined threshold values.
Automated insulin delivery is made possible through combining Medtronic insulin pump and continuous glucose monitoring technology. Some user interaction required. Individual responses to the treatment can and do vary Consult a healthcare professional to see if this product is suitable for you.
The Guardian 4 sensor is intended for use with the Guardian 4 transmitter to monitor glucose levels in persons with diabetes where self-monitoring of blood glucose (SMBG) is indicated. The sensor is designed to replace fingerstick blood glucose (BG)readings for diabetes treatment decisions. The sensor is intended for insertion into persons ages 7 years and older. The sensor is intended for insertion into the back of the upper arm or the upper buttocks in persons ages 7 through 17 years. The sensor is intended for insertion into the back of the upper arm or the abdomen in persons ages 18 years and older.
References
^ Components sold separately.
1. Carlson, A.L. et al. Safety and Glycemic Outcomes During the MiniMed Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. DIABETES TECHNOLOGY & THERAPEUTICS. Vol 24, No. 3 (2022) 1-12.
2. Da Silva J, et al. Real-world Performance of the MiniMed™ 780G System: First Report of Outcomes from 4'120 Users. Diabetes Technol Ther. 2021; doi: 10.1089/dia.2021.0203
3. Data on file from CIP 321: Pivotal Trial (Age 7-75). N=283. 2020; 16 US sites.
4. Sherr J.L, et al. Poster A66 Journal of Diabetes Science and Technology 2021; 15(2) 397–477
5. Google Play and the Google Play logo are trademarks of Google LLC.
§ Compared to the MiniMedTM 770G system. Refers to SmartGuardTM feature. Some user interaction required.
* if sensor glucose (SG) values do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide.
** Refers to SmartGuard™ feature. Individual results may vary.
† At the time of manufacture and when the reservoir and tubing are properly inserted, your pump is waterproof. It is protected against the effects of being underwater to a depth of up to 12 feet (3.6 meters) for up to 24 hours. This is classified as IPX8 rating. See user guide for more details. The sensor and transmitter are water-resistant at 8 feet (2.4 meters) for up to 30 minutes. CGM readings may not be transmitted from the CGM to the pump while in water.
‡ Assumes 4 injections per day for 30 days and one infusion set change every seven days for extended infusion set.